
Inspection Process and Key Points for Gaomi Nitrile Gloves
1. Pre-Inspection Preparation
Document Review: Verify product specifications, PO requirements, and quality standards (e.g., ASTM D6319 for nitrile gloves).
Sampling Plan: Follow AQL (Acceptable Quality Level) standards (typically Level II, AQL 1.0/2.5 for major/minor defects).
Tools Preparation: Prepare calipers, weighing scale, tensile tester, visual inspection tools, and leak tester (water filling or air inflation method).

2. Visual Inspection
Surface Quality: Check for holes, tears, uneven thickness, stains, or foreign particles.
Molding & Trimming: Ensure smooth edges, no excessive flashing, or incomplete molding.
Printing & Marking: Verify logos, sizes, and certifications (e.g., CE, FDA) are legible and correct.

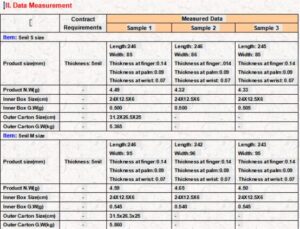
3. Dimensional & Weight Checks
Length/Width/Thickness: Measure with calipers per ASTM standards (e.g., thickness tolerance ±0.05mm).
Weight: Weigh random samples; ensure consistency (e.g., ±0.5g per glove).
4. Physical Performance Tests
Tensile Strength & Elongation: Test using a tensile machine (ASTM D412; typical requirements: tensile strength ≥14MPa, elongation ≥500%).
Puncture Resistance: Check per ASTM F1342 (e.g., puncture force ≥1.5N).
5. Special function test
Barcode Scan
Basic function check (Functions on product specification work )
Water loading test
Pull test
Smell check
Color shading check
Rub test
Carton drop test

6. Chemical & Material Safety
Certificates: Confirm nitrile material complies with FDA 21 CFR 177.2600 or EU 10/2011 for food contact (if required).
Allergen Check: Ensure no harmful accelerators (e.g., MBT, ZDBC) exceed limits.
7. Packaging & Labeling
Inner/Outer Packaging: Verify cleanliness, sealing integrity, and moisture-proof measures.
Label Accuracy: Check batch number, expiry date, size, and compliance markings.

8. Defect Classification
Critical Defects: Holes, chemical contamination (reject entire lot).
Major Defects: Thickness deviation, weak seams (AQL 1.0).
Minor Defects: Minor stains, printing errors (AQL 2.5).
9. Reporting
Inspection Report: Record defects, test data, and non-conformities with photos.
Disposition: Recommend approval/rejection based on AQL results.
Key Focus Areas:
Consistency: Thickness, color, and texture uniformity.
Durability: Tensile strength and leak resistance.
Safety: Compliance with FDA/CE and absence of toxins.



